HSPA1B (基因名), Heat shock 70 kDa protein 1B (蛋白名), hs71b_bovin.
产品名称:
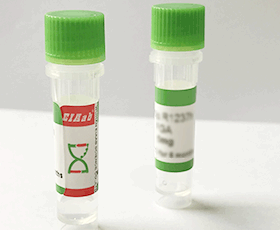
Bovine HSPA1B/ Heat shock 70 kDa protein 1B Recombinant Protein
热休克蛋白70蛋白1B
货号:
R8770b
商标:
EIAab®
监管等级:
别名:
Heat shock 70 kDa protein 2, HSP70.2, HSP70-2
序列号:
Q27965
来源:
E.coli
种属:
Bovine
标签:
His
纯度:
>90% by SDS-PAGE
浓度:
Reconstitution Dependent
形态:
Liquid
内毒素水平:
Please contact protein@eiaab.com The technician for more information.
应用:
存储缓冲液:
50mM NaH2PO4, 500mM NaCl Buffer with 500mM Imidazole, 10%glycerol(PH8.0)
存储:
Store at -20°C. (Avoid repeated freezing and thawing.)
研究领域:
-
R&D 技术数据
更多信息,请参阅手册,或联系我们的技术支持: tech@eiaab.com.
通用注释
亚单元:
Component of the CatSper complex. Identified in a IGF2BP1-dependent mRNP granule complex containing untranslated mRNAs. Interacts with CHCHD3, DNAJC7, IRAK1BP1, PPP5C and TSC2. Interacts with TERT; the interaction occurs in the absence of the RNA component, TERC, and dissociates once the TERT complex has formed. Interacts with METTL21A. Interacts with TRIM5 (via B30.2/SPRY domain). Interacts with PARK2. Interacts with FOXP3. Interacts with NOD2; the interaction enhances NOD2 stability. Interacts with DNAJC9 (via J domain). Interacts with ATF5; the interaction protects ATF5 from degradation via proteasome-dependent and caspase-dependent processes. Interacts with NAA10, HSP40, HSP90 and HDAC4. The acetylated form and the non-acetylated form interact with HOPX and STUB1 respectively. Interacts with NEDD1 and SMAD3. Interacts (via NBD) with BAG1, BAG2, BAG3 and HSPH1/HSP105.
功能:
Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation. Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle. Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling. Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation.
亚细胞位置:
Cytoplasm
Cytoplasm
Cytoskeleton
Microtubule organizing center
Centrosome
Localized in cytoplasmic mRNP granules containing untranslated mRNAs.
数据库链接
UniGene:
SMR:
STRING:
KEGG:
Pfam:
Uniprot:
该产品尚未在任何出版物中被引用。
[1].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白是否是无菌的?
蛋白试剂瓶和蛋白保存液是经过高压灭菌的,但也不能保证蛋白是完全无菌的。如果要求蛋白是无菌的,可以用0.2微米的滤器对蛋白进行过滤。
[2].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白的保存缓冲液是什么?
纯化后的蛋白保存在PBS(58mM Na2HPO4, 17mM NaH2PO4, 68mM NaCl, pH7.4)里,并往里面加入500mM咪唑和10%甘油。
[3].
怎样确定牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白的浓度?
蛋白浓度的确定没有一个统一的标准,这主要取决于蛋白的氨基酸序列。伊艾博是根据不同测试的组合来测定蛋白浓度。考马斯亮蓝法、BCA法、氨基酸序列和氨基酸全序列分析法等都用来测定蛋白浓度。
[4].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白蛋白保存条件是怎样的?
蛋白应保存在 -20℃或 -80℃条件下,为了避免反复冻融,可以将蛋白分装成小份保存。
[5].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白是否可以用于活体实验?
重组蛋白没有用于任何的活体实验,因此蛋白的活性和半衰期是不确定的。
[6].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白的保质期是多久?
在适当的保存条件下,从购买之日起蛋白可以稳定保存6-12个月。适当的保存条件是:蛋白保存在-20°C o或 -80℃,保证蛋白的保存浓度高于0.1mg/ml,限制蛋白反复冻融的次数。我们公司常规的质量检测保证所有产品在销售时都有可接受的生物活性。但是我们不能控制终端用户蛋白的保存条件。如果产品在有效期内出现问题,请联系我们的技术支持。
[7].
你们蛋白和抗体的报价是怎么样的?
我们将根据你需要的蛋白和抗体的大小进行报价。
[8].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白是否能够提供蛋白片段?
我们现有的人的蛋白的序列可以有很多。你可以选择你感兴趣的靶向部分,我们将会按您的需求提供蛋白和抗体。
[9].
牛热休克蛋白70蛋白1B(HSPA1B)重组蛋白的货期或发货时间一般是多长?
具体指标的货期需要确定。最快一周,最长可能一个月。
反馈墙
评论数 : 0
所有用户
所有用户
默认排序
默认排序
最近
早期
目前还没有评论。



通知
规格
数量
单价 (¥)
小计 1 (¥)
小计 2:
¥

规格
数量
单价 (¥)




 验证序列:
验证序列:




 折扣:
折扣: