HSP90AA1 (基因名), Heat shock protein HSP 90-alpha (蛋白名), hs90a_human.
产品名称:
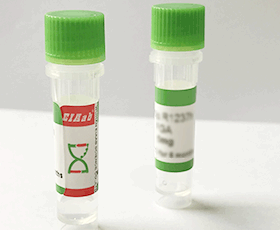
Human HSP90AA1/ Heat shock protein HSP 90-alpha Recombinant Protein
热休克蛋白HSP90-α
货号:
R0863h
商标:
EIAab®
监管等级:
别名:
Heat shock 86 kDa, Lipopolysaccharide-associated protein 2, Renal carcinoma antigen NY-REN-38, HSP 86, LAP-2, HSP90A, HSPC1, HSPCA
序列号:
P07900
来源:
E.coli
种属:
Human
标签:
His
序列:
682-732aa
预估分子量:
5.61 kDa (monomer)
纯度:
Greater than 92% by SDS-PAGE
浓度:
Reconstitution Dependent
形态:
Liquid
内毒素水平:
Please contact protein@eiaab.com The technician for more information.
应用:
存储缓冲液:
50mM NaH2PO4, 500mM NaCl Buffer with 500mM Imidazole, 10%glycerol(PH8.0)
存储:
Store at -20°C. (Avoid repeated freezing and thawing.)
研究领域:
Epigenetics
R&D 技术数据
更多信息,请参阅手册,或联系我们的技术支持: tech@eiaab.com.
基因位点
HSP90AA1 Gene in genomic location: bands according to Ensembl, locations according to GeneLoc (and/or Entrez Gene and/or Ensembl if different)

通用注释
亚单元:
Homodimer (PubMed:7588731, PubMed:8289821, PubMed:18400751). Identified in NR3C1/GCR steroid receptor-chaperone complexes formed at least by NR3C1, HSP90AA1 and a variety of proteins containing TPR repeats such as FKBP4, FKBP5, PPID, PPP5C or STIP1 (PubMed:15383005, PubMed:9195923). Interacts with TOM34 (PubMed:9660753). Interacts with TERT; the interaction, together with PTGES3, is required for correct assembly and stabilization of the TERT holoenzyme complex (PubMed:11274138, PubMed:9817749). Interacts with CHORDC1 and DNAJC7 (PubMed:12853476, PubMed:19875381). Interacts with STUB1 and UBE2N; may couple the chaperone and ubiquitination systems (PubMed:16307917, PubMed:27353360). Interacts (via TPR repeat-binding motif) with PPP5C (via TPR repeats); the interaction is direct and activates PPP5C phosphatase activity (PubMed:15383005, PubMed:15577939, PubMed:16531226, PubMed:27353360). Following LPS binding, may form a complex with CXCR4, GDF5 and HSPA8 (PubMed:11276205). Interacts with KSR1 (PubMed:10409742). Interacts with co-chaperone CDC37 (via C-terminus); the interaction inhibits HSP90AA1 ATPase activity (PubMed:23569206, PubMed:27353360). May interact with NWD1 (PubMed:24681825). Interacts with FNIP1 and FNIP2; the interaction inhibits HSP90AA1 ATPase activity (PubMed:17028174, PubMed:27353360). Interacts with AHSA1; the interaction activates HSP90AA1 ATPase activity (PubMed:12604615, PubMed:27353360). Interacts with FLCN in the presence of FNIP1. Interacts with HSP70, STIP1 and PTGES3 (PubMed:27353360). Interacts with SMYD3; this interaction enhances SMYD3 histone-lysine N-methyltransferase (PubMed:15235609, PubMed:25738358). Interacts with SGTA (via TPR repeats) (PubMed:15708368). Interacts with TTC1 (via TPR repeats) (PubMed:15708368). Interacts with HSF1 in an ATP-dependent manner (PubMed:11583998. PubMed:26517842). Interacts with MET; the interaction suppresses MET kinase activity. Interacts with ERBB2 in an ATP-dependent manner; the interaction suppresses ERBB2 kinase activity. Interacts with HIF1A, KEAP1 and RHOBTB2 (PubMed:26517842). Interacts with HSF1; this interaction is decreased in a IER5-dependent manner, promoting HSF1 accumulation in the nucleus, homotrimerization and DNA-binding activities (PubMed:26754925). Interacts with STUB1 and SMAD3 (PubMed:24613385). Interacts with HSP90AB1; interaction is constitutive (PubMed:20353823).
功能:
Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:15577939, PubMed:15937123, PubMed:27353360). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385).
亚细胞位置:
Cytoplasm
Melanosome
Cell membrane
Identified by mass spectrometry in melanosome fractions from stage I to stage IV.
该产品尚未在任何出版物中被引用。
[1].
"A proteomic investigation of ligand-dependent HSP90 complexes reveals CHORDC1 as a novel ADP-dependent HSP90-interacting protein."
[4].
"The HSP90 family of genes in the human genome: insights into their divergence and evolution."
[5].
"Human protein phosphatase 5 dissociates from heat-shock proteins and is proteolytically activated in response to arachidonic acid and the microtubule-depolymerizing drug nocodazole."
[6].
"S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities."
[7].
"Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system."
[9].
"Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90."
[10].
"Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin."
[1].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白是否是无菌的?
蛋白试剂瓶和蛋白保存液是经过高压灭菌的,但也不能保证蛋白是完全无菌的。如果要求蛋白是无菌的,可以用0.2微米的滤器对蛋白进行过滤。
[2].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白的保存缓冲液是什么?
纯化后的蛋白保存在PBS(58mM Na2HPO4, 17mM NaH2PO4, 68mM NaCl, pH7.4)里,并往里面加入500mM咪唑和10%甘油。
[3].
怎样确定人热休克蛋白HSP90-α(HSP90AA1)重组蛋白的浓度?
蛋白浓度的确定没有一个统一的标准,这主要取决于蛋白的氨基酸序列。伊艾博是根据不同测试的组合来测定蛋白浓度。考马斯亮蓝法、BCA法、氨基酸序列和氨基酸全序列分析法等都用来测定蛋白浓度。
[4].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白蛋白保存条件是怎样的?
蛋白应保存在 -20℃或 -80℃条件下,为了避免反复冻融,可以将蛋白分装成小份保存。
[5].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白是否可以用于活体实验?
重组蛋白没有用于任何的活体实验,因此蛋白的活性和半衰期是不确定的。
[6].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白的保质期是多久?
在适当的保存条件下,从购买之日起蛋白可以稳定保存6-12个月。适当的保存条件是:蛋白保存在-20°C o或 -80℃,保证蛋白的保存浓度高于0.1mg/ml,限制蛋白反复冻融的次数。我们公司常规的质量检测保证所有产品在销售时都有可接受的生物活性。但是我们不能控制终端用户蛋白的保存条件。如果产品在有效期内出现问题,请联系我们的技术支持。
[7].
你们蛋白和抗体的报价是怎么样的?
我们将根据你需要的蛋白和抗体的大小进行报价。
[8].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白是否能够提供蛋白片段?
我们现有的人的蛋白的序列可以有很多。你可以选择你感兴趣的靶向部分,我们将会按您的需求提供蛋白和抗体。
[9].
人热休克蛋白HSP90-α(HSP90AA1)重组蛋白的货期或发货时间一般是多长?
具体指标的货期需要确定。最快一周,最长可能一个月。
反馈墙
评论数 : 0
所有用户
所有用户
默认排序
默认排序
最近
早期
目前还没有评论。



通知
规格
数量
单价 (¥)
小计 1 (¥)
小计 2:
¥

规格
数量
单价 (¥)





 验证序列:
验证序列:




 折扣:
折扣: