VIL1 (基因名), Villin-1 (蛋白名), vili_human.
产品名称:
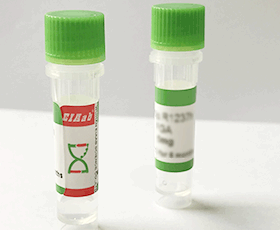
Human VIL1/ Villin-1 Recombinant Protein
Villin-1
货号:
R0193h
商标:
EIAab®
监管等级:
别名:
VIL
序列号:
P09327
来源:
E.coli
种属:
Human
标签:
His
纯度:
>90% by SDS-PAGE
浓度:
Reconstitution Dependent
形态:
Liquid
内毒素水平:
Please contact protein@eiaab.com The technician for more information.
应用:
存储缓冲液:
50mM NaH2PO4, 500mM NaCl Buffer with 500mM Imidazole, 10%glycerol(PH8.0)
存储:
Store at -20°C. (Avoid repeated freezing and thawing.)
研究领域:
-
R&D 技术数据
更多信息,请参阅手册,或联系我们的技术支持: tech@eiaab.com.
基因位点
VIL1 Gene in genomic location: bands according to Ensembl, locations according to GeneLoc (and/or Entrez Gene and/or Ensembl if different)

通用注释
亚单元:
Monomer. Homodimer; homodimerization is necessary for actin-bundling. Associates with F-actin; phosphorylation at tyrosine residues decreases the association with F-actin. Interacts (phosphorylated at C-terminus tyrosine phosphorylation sites) with PLCG1 (via the SH2 domains). Interacts (phosphorylated form) with PLCG1; the interaction is enhanced by hepatocyte growth factor (HGF).
功能:
Epithelial cell-specific Ca(2+)-regulated actin-modifying protein that modulates the reorganization of microvillar actin filaments. Plays a role in the actin nucleation, actin filament bundle assembly, actin filament capping and severing. Binds phosphatidylinositol 4,5-bisphosphate (PIP2) and lysophosphatidic acid (LPA); binds LPA with higher affinity than PIP2. Binding to LPA increases its phosphorylation by SRC and inhibits all actin-modifying activities. Binding to PIP2 inhibits actin-capping and -severing activities but enhances actin-bundling activity. Regulates the intestinal epithelial cell morphology, cell invasion, cell migration and apoptosis. Protects against apoptosis induced by dextran sodium sulfate (DSS) in the gastrointestinal epithelium. Appears to regulate cell death by maintaining mitochondrial integrity. Enhances hepatocyte growth factor (HGF)-induced epithelial cell motility, chemotaxis and wound repair. Upon S.flexneri cell infection, its actin-severing activity enhances actin-based motility of the bacteria and plays a role during the dissemination.
亚细胞位置:
Cytoplasm
Cytoskeleton
Cell projection
Lamellipodium
Cell projection
Ruffle
Cell projection
Microvillus
Cell projection
Filopodium tip
Cell projection
Filopodium
Relocalized in the tip of cellular protrusions and filipodial extensions upon infection with S.flexneri in primary intestinal epithelial cells (IEC) and in the tail-like structures forming the actin comets of S.flexneri. Redistributed to the leading edge of hepatocyte growth factor (HGF)-induced lamellipodia (By similarity). Rapidly redistributed to ruffles and lamellipodia structures in response to autotaxin, lysophosphatidic acid (LPA) and epidermal growth factor (EGF) treatment.
该产品尚未在任何出版物中被引用。
[1].
"Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity."
[2].
"Differential effects of lysophosphatidic acid and phosphatidylinositol 4,5-bisphosphate on actin dynamics by direct association with the actin-binding protein villin."
[3].
"Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia."
[5].
"Obligatory role for phospholipase C-gamma(1) in villin-induced epithelial cell migration."
[6].
"Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders."
[7].
"Interaction of phospholipase C-gamma1 with villin regulates epithelial cell migration."
[8].
"Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton."
[9].
"Solution structures of the C-terminal headpiece subdomains of human villin and advillin, evaluation of headpiece F-actin-binding requirements."
[10].
"Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin."
[1].
人Villin-1(VIL1)重组蛋白是否是无菌的?
蛋白试剂瓶和蛋白保存液是经过高压灭菌的,但也不能保证蛋白是完全无菌的。如果要求蛋白是无菌的,可以用0.2微米的滤器对蛋白进行过滤。
[2].
人Villin-1(VIL1)重组蛋白的保存缓冲液是什么?
纯化后的蛋白保存在PBS(58mM Na2HPO4, 17mM NaH2PO4, 68mM NaCl, pH7.4)里,并往里面加入500mM咪唑和10%甘油。
[3].
怎样确定人Villin-1(VIL1)重组蛋白的浓度?
蛋白浓度的确定没有一个统一的标准,这主要取决于蛋白的氨基酸序列。伊艾博是根据不同测试的组合来测定蛋白浓度。考马斯亮蓝法、BCA法、氨基酸序列和氨基酸全序列分析法等都用来测定蛋白浓度。
[4].
人Villin-1(VIL1)重组蛋白蛋白保存条件是怎样的?
蛋白应保存在 -20℃或 -80℃条件下,为了避免反复冻融,可以将蛋白分装成小份保存。
[5].
人Villin-1(VIL1)重组蛋白是否可以用于活体实验?
重组蛋白没有用于任何的活体实验,因此蛋白的活性和半衰期是不确定的。
[6].
人Villin-1(VIL1)重组蛋白的保质期是多久?
在适当的保存条件下,从购买之日起蛋白可以稳定保存6-12个月。适当的保存条件是:蛋白保存在-20°C o或 -80℃,保证蛋白的保存浓度高于0.1mg/ml,限制蛋白反复冻融的次数。我们公司常规的质量检测保证所有产品在销售时都有可接受的生物活性。但是我们不能控制终端用户蛋白的保存条件。如果产品在有效期内出现问题,请联系我们的技术支持。
[7].
你们蛋白和抗体的报价是怎么样的?
我们将根据你需要的蛋白和抗体的大小进行报价。
[8].
人Villin-1(VIL1)重组蛋白是否能够提供蛋白片段?
我们现有的人的蛋白的序列可以有很多。你可以选择你感兴趣的靶向部分,我们将会按您的需求提供蛋白和抗体。
[9].
人Villin-1(VIL1)重组蛋白的货期或发货时间一般是多长?
具体指标的货期需要确定。最快一周,最长可能一个月。
反馈墙
评论数 : 0
所有用户
所有用户
默认排序
默认排序
最近
早期
目前还没有评论。



通知
规格
数量
单价 (¥)
小计 1 (¥)
小计 2:
¥

规格
数量
单价 (¥)
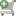





 验证序列:
验证序列:




 折扣:
折扣: